



| Lentivirus Packaging | Titer | TAT |
| Over expression Lentivirus | ≥1 E+8 TU/mL(Standard) ≥5 E+8 TU/mL ≥1 E+9 TU/mL |
15~25 days,including gene synthesis cycles |
| RNAi Lentivirus(single target) | ≥1 E+8 TU/mL ≥1 E+9 TU/mL |
|
| RNAi Lentivirus Set(three target) | ≥1 E+8 TU/mL | |
| sgRNA Lentivirus(single target) | ≥1 E+8 TU/mL ≥1 E+9 TU/mL |
|
| sgRNA Lentivirus Set(three target) | ≥1 E+8 TU/mL |
| Adeno-AssociatedVirus(AAV) Packaging | Volume | TAT |
| Ultra-purified ssAAV packaging (normal scale serotypes) | 5E+11 VGS;1E+12 VGS; 2E+12 VGS;5E+12 VGS; 1E+13 VGS;2E+13 VGS; 5E+13 VGS;1E+14 VGS |
12~18 business days,excluding gene synthesis cycles |
| Ultra-purified ssAAV packaging (Low scale serotypes) | 2E+12 VGS;5E+12 VGS; 1E+13 VGS;2E+13 VGS; 5E+13 VGS;1E+14 VGS |
|
| Ultra-purified scAAV packaging | 5E+11 VGS;1E+12 VGS; 2E+12 VGS;5E+12 VGS; 1E+13 VGS;2E+13 VGS; 5E+13 VGS;1E+14 VGS; 2E+14 VGS |
| Adeno-AssociatedVirus(ADV) Packaging | Volume | TAT |
| Ultra-purified scADV packaging | 1E+10PFU;5E+10PFU;1E+11PFU | 55 business days,excluding gene synthesis cycles |
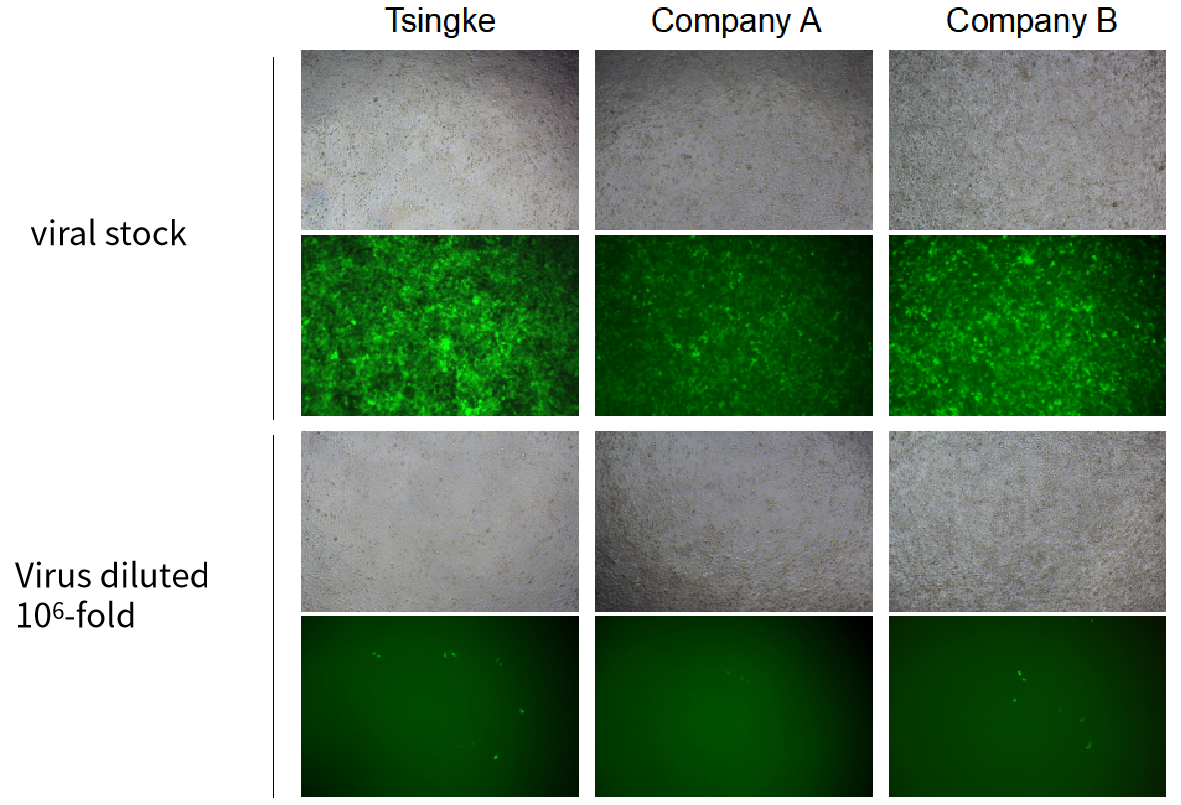
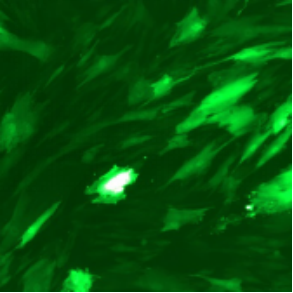
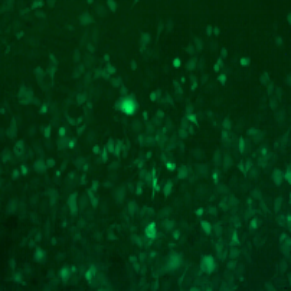
| Adenovirus |
ADV-CMV-EGFP | |||||
| MOI | 1000 | |||||
| Cell | 231 | hBMSCS | U251 | MEF | A549 | T24 |
| Virus Effect | 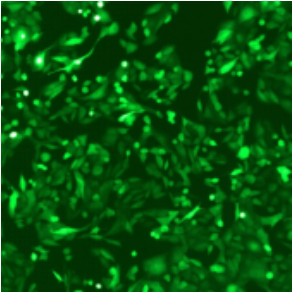 |
 |
 |
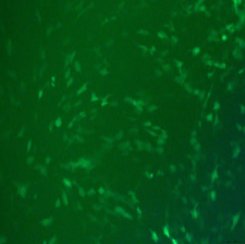 |
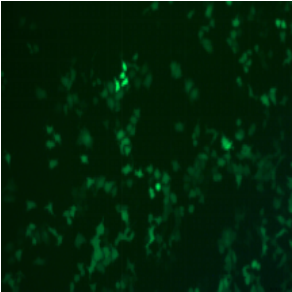 |
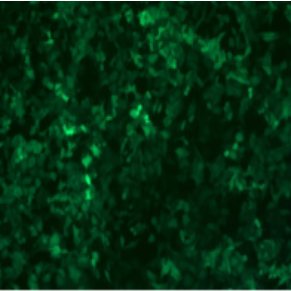 |
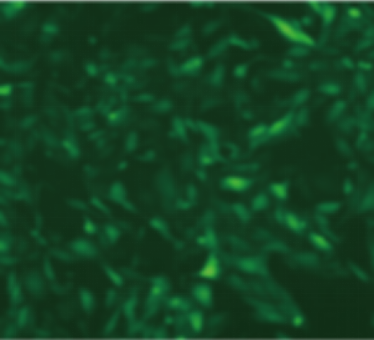
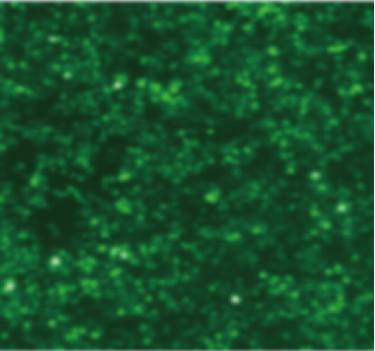
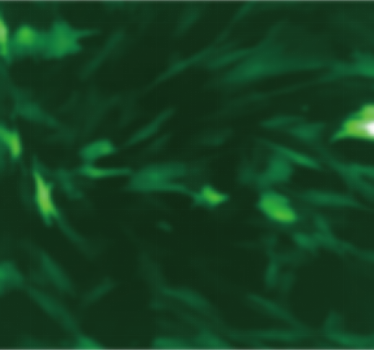
| Adeno-associated virus |
ssAAV-CMV-EGFP-PolyA(AAV6) | scAAV-CMV-EGFP-PolyA(AAVDJ) | scAAV-CMV-EGFP-PolyA (AAVDJ) | scAAV-CMV-EGFP-PolyA (AAVDJ) |
| MOI | 1000000 | 4000000 | 4000000 | 4000000 |
| Cell | 231 | 231 | DC2.4 | hBMSCs |
| Virus Effect | 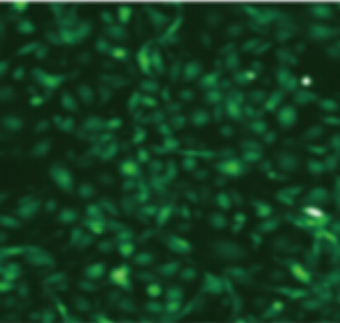 |
 |
 |
 |