Tsingke has established high-standard production facilities to ensure manufacturing strictly adheres to relevant regulations and standards, guaranteeing high-quality and safe products.
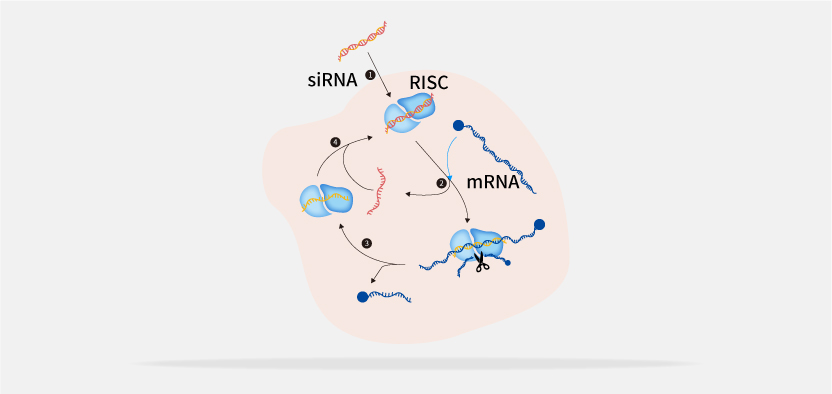
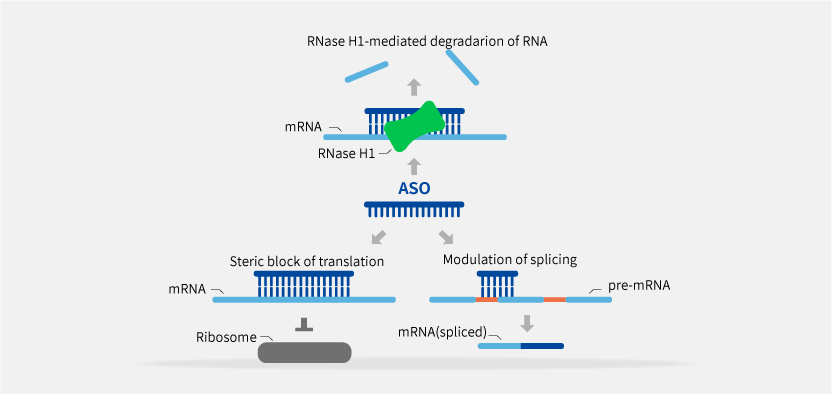
Tsingke provides comprehensive solutions for small nucleic acid drug discovery, including target screening, drug design and synthesis, identification and validation of cell-level candidate drugs, toxicity evaluation, and optimization.
In CRO design and development, Tsingke has designed over 10,000 gene-specific siRNAs and has a rich talent pool, delivering over 100,000 siRNAs per year.
In assay development, Tsingke has pre-stocked over 13,000 overexpression genes, 20,000 shRNA plasmids, and 5,000 sgRNA plasmids, and has validated 5,000 ready-to-use primers. Tsingke offers high-quality, efficient qRT-PCR and Western blotting services to accelerate target screening and validation.
Tsingke supports IND application services, including regulatory consulting, project management, pharmaceutical documentation preparation, and IND application assistance, helping customers navigate the IND application process smoothly.