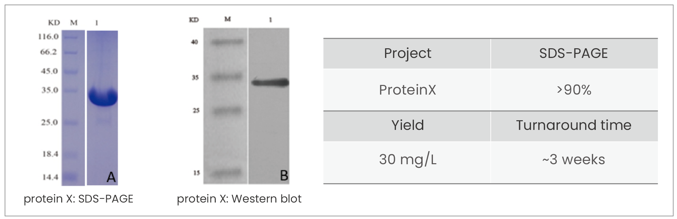
Product&Services
Tsynth™ Gene Synthesis
Oligo Synthesis
Protein Expression
Antibody Service
Gene Regulation
TSINGKE Gene Factory - Sequencing