
Service Details
|
Service Name |
Expression system |
Service Code |
Volume |
Turnaround time |
|
Recombinant Antibody Expression Service |
HEK293/CHO |
Tsingke-006-3 |
10 mL |
1~2 weeks |
|
Tsingke-006-4 |
20 mL |
|||
|
Tsingke-006-5 |
30 mL |
|||
|
Tsingke-006-6 |
40 mL |
|||
|
Tsingke-006-7 |
50 mL |
|||
|
Tsingke-006-8 |
100 mL |
|||
|
Tsingke-006-9 |
200 mL |
|||
|
Tsingke-006-10 |
500 mL |
|||
|
Tsingke-006-11 |
1000 mL |
|||
|
Tsingke-006-12 |
>1 L |
|
Deliverables |
Delivery Standard |
|
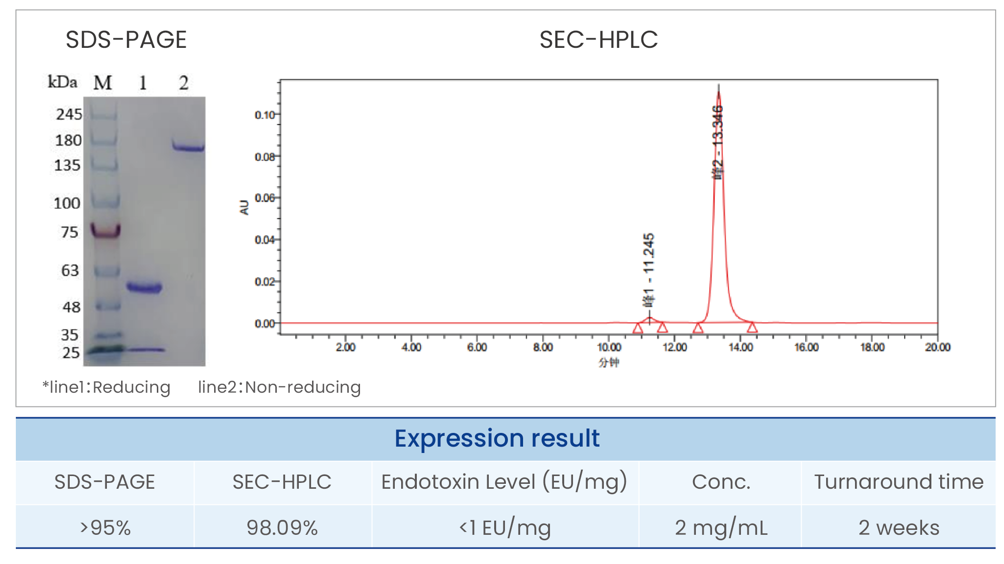
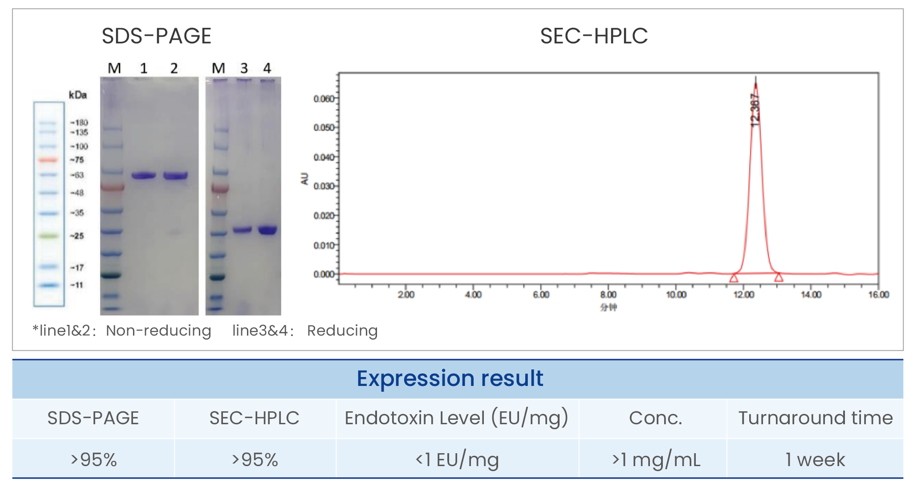
Purified antibodies; COA Report(electronic); Optional: |
SDS-PAGE&SEC-HPLC >95%; Endotoxin level <1 EU/mg. |