|
Research Grade |
||
|
Volume |
*Turnaround Time(Natural Day) |
Deliverables |
|
100 μg |
5 |
Prepared lyophilized plasmid DNA; Sequencing map (.abl file); COA Report(electronic). |
|
200 μg |
6 | |
|
500 μg |
8 | |
|
1 mg |
8 | |
|
2 mg |
9 | |
|
3 mg |
10 | |
|
4 mg |
11 | |
|
5 mg |
12 | |
|
10 mg |
15 | |
|
20 mg |
16 | |
|
50 mg |
17 | |
|
100 mg |
21 | |
|
Quality Control |
|||
|
Research Grade |
Items |
Method |
Specifications |
|
Appearance |
Visualinspection |
Colorless Liquid of Pure Transparency |
|
|
A260/280 ratio |
UV Absorbance |
1.8~2.0 |
|
|
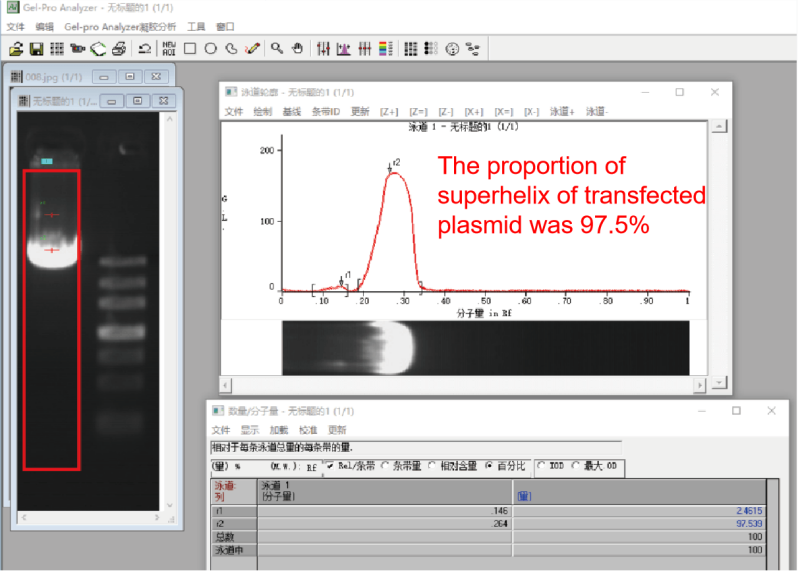
Supercoil content |
Agarose gel electrophoresis |
>50% |
|
|
Concentration |
UV Absorbance |
90%~110% |
|
|
Residual DNA |
Agarose gel electrophoresis |
Not visible |
|
|
Residual RNA |
Agarose gel electrophoresis |
Not visible |
|
|
Restriction enzyme analysis |
Agarose gel electrophoresis |
Adjustable according to requirement |
|
|
Sequence verification |
Sanger sequencing |
Consistent with the confirmed |
|
|
Transfection Grade |
|||
|
Endotoxin |
Volume |
*Turnaround Time(Natural Day) |
Deliverables |
|
<0.1 Eu/ μg |
100 μg |
5 |
Prepared lyophilized plasmid DNA; |
|
200 μg |
6 | ||
|
500 μg |
8 | ||
|
<0.01 Eu/ μg |
1 mg |
8 | |
|
2 mg |
9 | ||
|
3 mg |
10 | ||
|
4 mg |
11 | ||
|
5 mg |
12 | ||
|
<0.005 Eu/ μg |
10 mg |
15 | |
|
20 mg |
17 | ||
|
50 mg |
19 | ||
|
100 mg |
22 | ||
|
Quality Control |
|||
|
Transfection Grade |
Items |
Method |
Specifications |
|
Appearance |
Visual inspection |
Colorless Liquid of Pure Transparency |
|
|
A260/280 ratio |
UV Absorbance |
1.8~2.0 |
|
|
Supercoil content |
Agarose gel electrophoresis |
>85% |
|
|
Concentration |
UV Absorbance |
90%~110% |
|
|
Residual DNA |
Agarose gel electrophoresis |
Not visible |
|
|
Residual RNA |
Agarose gel electrophoresis |
Not visible |
|
|
Restriction enzyme analysis |
Agarose gel electrophoresis |
Adjustable according to requirement |
|
|
Sequence verification |
Sanger sequencing |
Consistent with the confirmed plasmid sequence |
|
|
Endotoxin |
Limulus amebocyte lysate (LAL) test |
Endotoxin ≤ Standard |
|
|
Exogenous Contamination Detection |
NGS(Depth>30×) |
Genomic DNA < 1% |
|